Pediatric acute lymphoblastic leukemia (ALL) patients have had significantly improved outcomes over the last few decades with overall survival (OS) rates exceeding 90%. Several factors, including the incorporation of asparaginase therapy in chemotherapy regimens of pediatric and AYA patients with ALL have played a critical role in improving outcomes. Asparaginase is a bacteria-derived enzyme which depletes the non-essential amino acid asparagine and leads to subsequent apoptosis in lymphoblastic leukemia cells. One of the main limitations of the frontline pegaspargase (PEG) chemotherapy is the development of hypersensitivity reactions and subsequent development of anti-asparaginase neutralizing antibodies that lead to PEG inactivation. When hypersensitivity reactions to PEG occur, patients are often switched to alternative forms of asparaginase, such as that isolated from Erwinia chrysanthemi. A prospective multi-institutional study initiated by Children's Hospital of Orange County (CHOC) and conducted at 6 pediatric cancer centers: 1) evaluated the efficacy of premedicating pediatric and AYA patients with B or T-ALL or lymphoblastic lymphoma (LBL) receiving PEG with antihistamines (H1 and H2 blockers), and 2) performed therapeutic drug monitoring to better identify patients with true hypersensitivity reactions versus those with non-antibody mediated infusion reactions with the goal of reducing incidences of substitution to Erwinia asparaginase. This study also aimed to characterize the pharmacogenomics which may be driving differences in asparaginase hypersensitivity to better understand which single nucleotide polymorphisms (SNPs) are involved in the immunogenicity of PEG and whether certain populations are more at risk for hypersensitivity. Here, we report preliminary evidence showing an association between a combination of 3 SNPs in 3 genes and incidence of hypersensitivity to PEG.
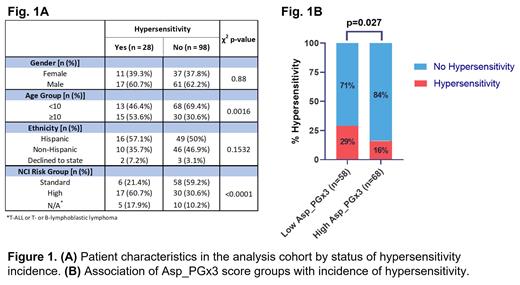
The analysis cohort consisted of 126 non-Down syndrome patients premedicated prior to PEG treatment. In this cohort, the median age was 7.7 years, 48 were female (38%) and 78 male (62%), 65 patients identified as Hispanic (52%), 56 as non-Hispanic (44%) and 5 declined to state (4%). Overall, 28 patients had a hypersensitive reaction to PEG within 15 minutes of infusion. Patient characteristics by hypersensitivity incidence are summarized in Fig 1A, with age and National Cancer Institute (NCI) risk groups being the factors that significantly differ by hypersensitivity. DNA from all 126 patients was used to genotype for 6 SNPs in 5 genes involved in pathways implicated in hypersensitivity to PEG. We tested each SNP genotype in 3 modes of inheritance (additive, dominant, and recessive) in all possible combinations of up to two SNPs per model using Cox-proportional hazard models for association with incidences of hypersensitivity post-infusion in 126 patients for whom clinical data was available. We then performed 1000 permutation tests for each model to determine the likelihood of obtaining them falsely. Models were ordered according to their Bayesian Information Criterion (BIC) and weight in favor of each model and those with least BIC and a 1000 permutation p<0.05 were selected for development of Asp_PGx3 score. This was defined by adding the genotype scores of 3 SNPs in 3 genes ( ARHGAP28, CNOT3, and NFATC2), accounting for the mode of inheritance and the direction of their association with outcome. This score ranged from -5 to 0, with patients with score <0 stratified as low score and those with scores of 0 as high score groups. A significantly higher proportion of patients in the low Asp_PGx3 score group (29%) had an incidence of PEG hypersensitivity compared to those in the high Asp_PGx3 score group (16%) (Fig 1B).
In conclusion, we show a combination of 3 SNPs (in ARHGAP28, CNOT3, and NFATC2) associated with PEG hypersensitivity in a cohort of 126 pediatric and AYA ALL/LBL patients. Instead of a single SNP association approach, identifying combinations of variations in pathway specific genes provides a more robust means to predict drug response. Our preliminary results provide a rationale for identification of genome level variations in this cohort and association analysis to establish clinically relevant pharmacogenomics score to optimize PEG treatment.
Funding for this study was provided by CHOC PSF Tithe Grant, CHOC Hyundai Cancer Institute Research Grant, and Servier Pharmaceuticals.
Disclosures
Agrawal:yMabs Therapeutics: Membership on an entity's Board of Directors or advisory committees, Other: Scientific Advisory Board. Apsel Winger:Novartis: Research Funding. Huynh:Servier: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal